EASY
Chemistry
IMPORTANT
Earn 100
When water is dropwise added into solution, initially brown colouration appears, further on adding excess water brown colouration disappears due to formation of colourless compound predict oxidation state of central atom in compound .
50% studentsanswered this correctly

Important Questions on The p-Block Elements
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
How many of the following reactions (not balanced eqn) undergo disproportionation reaction?
i)
ii)
iii)
iv)
v)
vi)
vii)
viii)
MEDIUM
Chemistry
IMPORTANT
Compound and are mixed in molar ratio. The mixture was subjected to ammonium molybdite test for phosphate estimation. The results of the test yielded a phosphate concentration of for the mixture. What is the concentration of compound in in the mixture?
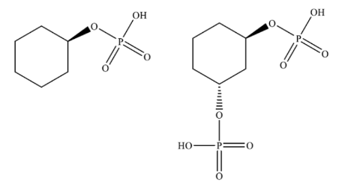
MEDIUM
Chemistry
IMPORTANT
i)
ii)
iii)
iv)
v)
vi)
vii)
viii)
EASY
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
