When a bit of baking soda is added to vinegar in a test tube, a hissing sound is heard and several bubbles occur. The test tube is closed and attached by a glass tube to another test tube containing lime water. The lime water slowly turns milky in the process. This is shown in the following experimental setup.
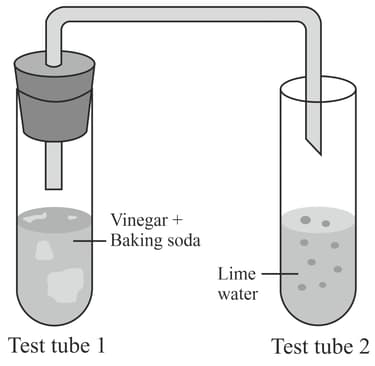
Which of the following can be considered as the indicator of a chemical reaction in this experiment?

Important Questions on Develop and use a model to describe how the total number of atoms does not change in a chemical reaction and thus mass is conserved.
The reaction of the iron nail with copper sulphate solution is a _____ reaction.
Calcium oxide reacts vigorously with water to produce slaked lime.
This reaction can be classified as:
(A) Combination reaction (B) Exothermic reaction
(C) Endothermic reaction (D) Oxidation reaction
Which of the following is the correct answer?
(a) Identify the type of reaction and the gas X.
(b) Write balanced chemical equation of the reaction.
(c) Write the range of aqueous solution of the gas X.
In a double displacement reaction such as the reaction between sodium sulphate solution and barium chloride solution:
(A) exchange of atoms takes place.
(B) exchange of ions takes place.
(C) a precipitate is produced.
(D) an insoluble salt is produced.
The correct option is:
The reaction of the burning of carbon in oxygen is represented by equation
When 9.0 g of solid carbon is burnt in 16.0 g of oxygen gas the mass of carbon dioxide gas formed would be:
(Note: Atomic mass of C-12,o u, O=16.0 u)
Write a balanced chemical equation for the following:
- Reaction of lead nitrate solution with ammonium hydroxide.
When hydrogen sulphide gas is passed through a blue solution of copper sulphate, a black precipitate of copper sulphide is obtained and the sulphuric acid so formed remains in the solution. The reaction is an example of a :
A metal compound A reacts with dilute hydrochloric acid to produce effervescence. The gas evolved extinguishes a burning candle.
Write a balanced equation for the reaction if one of the compounds formed is calcium chloride.

