EASY
Earn 100
When a bottle of dry ammonia and a bottle of dry HCI connected through a long tube are opened simultaneously at both ends, at first.
(a)A white ring is formed at the centre of the tube
(b)A white ring is formed near the ammonia bottle
(c)Entire length of tube turns white
(d)A white ring is formed near HCI bottle
50% studentsanswered this correctly
Important Questions on States of Matter: Gases and Liquids
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
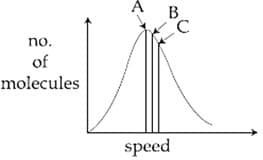
Root mean square speed most proable speed Average speed
HARD
EASY
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
EASY
EASY

