MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Earn 100
When a certain amount of ethylene was combusted, heat was evolved. If heat of combustion of ethylene is, determine the volume of (at ) that entered into the reaction.

Important Questions on Thermodynamics & Thermochemistry
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Given the following reactions:
I:
II:
Which amongst and is more stable?
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Enthalpy of polymerisation of ethylene, as represented by the reaction, is per mole of ethylene. Given bond enthalpy of bond is , determine enthalpy of bond (in ).
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Substance can undergo decomposition to form two sets of products:
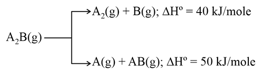
If the molar ratio of to is in a set of product gases, determine then the energy involved in the decomposition of mole of .
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
For the hypothetical reaction:
If and are and , respectively at is then determine at .
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
A molecule whose molar specific heat at high temperature, assuming ideal behaviour, is is:
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
The maximum amount of work produced by a heat engine operating between and , if of heat is absorbed from the hot reservoir, is:
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
The ratio of the heat capacities for one mole of a gas is . The gas is:
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
A cylinder of cooking gas in a household contains of butane. The thermochemical reaction for the combustion of butane is . If the household needs of energy per day, the cooking gas cylinder will last for about:
