EASY
JEE Main/Advance
IMPORTANT
Earn 100
When a certain amount of heat is supplied to a diatomic gas at constant volume, rise in temperature is When same heat is supplied to the gas at constant pressure, what is the rise in temperature?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
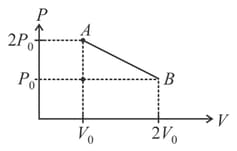
In the given diagram, if temperature at is then maximum temperature during the process is_____