When a liquid evaporates, the heat supplied to it is used partially to do work in overcoming the forces of cohesion between the molecules (the internal heat of vaporization) and partially to do work against the forces caused by external pressure (the external heat of vaporization). How to determine the external heat of vaporization from the graph representing the experimental isotherm of a real gas?

Important Questions on Molecular Physics and Thermodynamics
To demonstrate the transition to the critical state, a liquid (usually ethyl ether) is placed inside a small sealed thick-walled glass tube. The tube is then sealed off (Figure) and slowly heated. It is found that in the process of heating the boundary between the liquid and the vapour above the liquid rises and the meniscus becomes flatter (Figure). It is extremely difficult to observe the transition through the critical temperature because of intense convective fluxes, but the result is seen because at this temperature the meniscus disappears completely (Figure). Upon slowly cooling the tube it is found that at the same temperature the entire volume becomes cloudy, so that light cannot pass through the tube (Figure). If the temperature is lowered still further, the volume becomes transparent and there appears a meniscus, which separates the two phases. Explain the reasons for the observed phenomena.
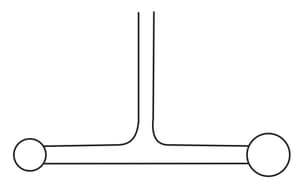
Two soap bubbles of different diameters are blown out using a -shaped pipe (see the figure). Will the diameters of the bubbles remain unchanged?
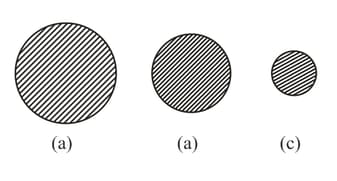
Three drops of different diameters are in the atmosphere of the vapor of the liquid from which the drops are formed. The pressure of the vapor is such that the drop with the medium diameter (Figure) is in equilibrium with the vapor. Is this equilibrium stable? How will the drops of the smaller (Figure) and the larger (Figure) diameters behave?
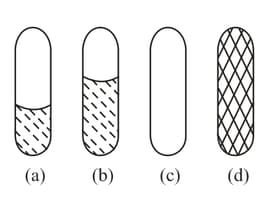
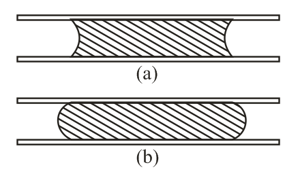
Two drops are placed between two parallel glass plates, a drop of water (Figure a) and a drop of mercury (Figure b). What forces act on the plates in each case?
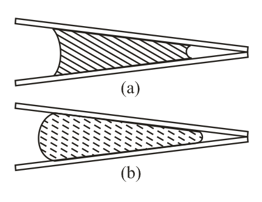
Inside two conical pipes there is a drop of water (Figure) and a drop of mercury (Figure). Where does each drop tend to move?