EASY
JEE Main/Advance
IMPORTANT
Earn 100
When a mixture containing phosphate is heated with conc. and ammonium molybdate solution, a canary yellow precipitate is formed. The formula of the yellow precipitate is:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Qualitative Analysis
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
: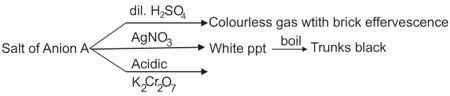
Shape of anion A will be:
EASY
JEE Main/Advance
IMPORTANT
Consider following reaction
Nitrite Acetic acid Thiourea
The formation of the product in the above reaction can be identified by
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT

The gas will show which of the following property?
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
In the following diagram bunsen flame the represents.
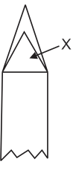
MEDIUM
JEE Main/Advance
IMPORTANT
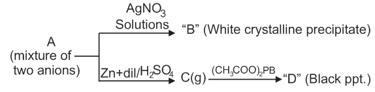
Then A may have:
