EASY
Earn 100
When a substance melts, the required energy is called a specific latent heat of vaporisation.
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Thermal Physics
EASY
MEDIUM
EASY
MEDIUM
EASY
EASY
MEDIUM
EASY
HARD
The figure below shows the variation of specific heat capacity () of a solid as a function of temperature (). The temperature is increased continuously from to at a constant rate. Ignoring any volume change, the following statement (s) is (are) correct to a reasonable approximation.
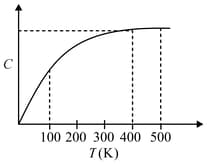
MEDIUM
HARD
(Specific heat of water is and the density of water is )
MEDIUM
EASY
MEDIUM
The triple-point of water is a standard fixed point in modern thermometry. Why? What is wrong in taking the melting point of ice and the boiling point of water as standard fixed points (as was originally done in the Celsius scale) ?
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
EASY

