MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
When a system is taken from state to state along the path , it is found that a quantity of heat, is absorbed by the system and work, is done by it. Along the path ,.
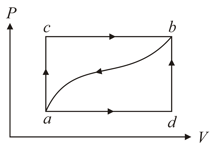
If , the value of will be,

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
JEE Main/Advance
IMPORTANT
When a system is taken from state to state along the path , it is found that a quantity of heat, is absorbed by the system and a work, is done by it. Along the path ,. Internal energy at is
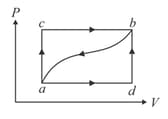
If , heat absorbed for the path is
MEDIUM
JEE Main/Advance
IMPORTANT
Ideal gas is taken through the process shown in figure:
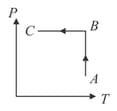
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
If molar heat capacity of the given process (as shown in figure) is , then,
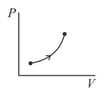
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
