EASY
Earn 100
When ammonium chloride is mixed with barium hydroxide in a beaker, it becomes cold.
Important Questions on Energy
HARD
EASY
HARD
It was found that the is decreased by in the presence of catalyst. If the rate remains unchanged, the activation energy for catalysed reaction is (Assume pre-exponential factor is same)
EASY
EASY
Consider the following energy profile
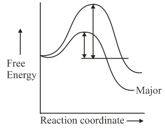
Which of the following reactant is most suited for the above energy profile?
MEDIUM
MEDIUM
MEDIUM
EASY
EASY
EASY
(Assume the pre-exponential factor & temperature to be same)
EASY
HARD
EASY
MEDIUM
EASY
During exothermic reaction, energy is _____.
HARD
HARD
EASY

