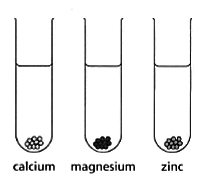
When calcium reacts with water, the reaction is:
- More vigorous (faster) than the reaction between zinc and water.
- Less vigorous (slower) than the reaction between magnesium and water.
Predict how the metals will react in hydrochloric acid by drawing bubbles in the test tubes.


Important Questions on Reactivity and Rates of Reaction
Aluminium is above iron in the reactivity series of metals.
Observations show that objects made from aluminium do not corrode as quickly as those made from iron. Use your research to explain why aluminium corrodes less quickly than expected to explain the observations.
Look at the diagram of the reactivity series — it shows the metals in order of reactivity. Read these statements:
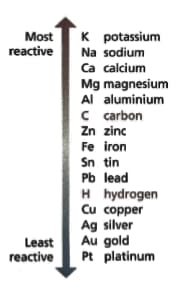
- Copper beads over years old have been found in Northern Iraq.
- The ancient Hittites of Asia Minor extracted iron in the .
- Sodium metal was first extracted in .
Describe the pattern you can see between a metal’s place in the reactivity series and its discovery.
Look at the diagram of the reactivity series — it shows the metals in order of reactivity. Read these statements:
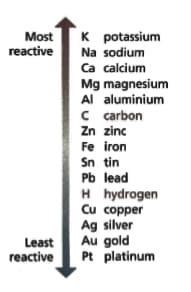
- Copper beads over years old have been found in Northern Iraq.
- The ancient Hittites of Asia Minor extracted iron in the .
- Sodium metal was first extracted in .
Suggest an explanation for the pattern of the metal's place in the reactivity series and it's discovery.
Priya and Carlos are investigating the reactivity of metals with different liquids.
Experiment : They add small pieces of the metals to water.
Experiment : They add small pieces of the metals to dilute hydrochloric acid.
What two variables should Priya and Carlos control?
Priya and Carlos are investigating the reactivity of metals with different liquids.
Experiment : They add small pieces of the metals to water.
Experiment : They add small pieces of the metals to dilute hydrochloric acid.
- Describe the two safety precautions they should take.
Priya and Carlos are investigating the reactivity of metals with different liquids.
Experiment : They add small pieces of the metals to water.
Experiment : They add small pieces of the metals to dilute hydrochloric acid.
Here are the results of the experiments:
| Metal |
Experiment : reaction with water |
Experiment : reaction with Hydrochloric acid. |
| A | Lots of bubbles | Lots of bubbles forming very quickly |
| B | No reaction | Some bubbles on the surface of metal |
| C | No reaction | No reaction |
| D | A few bubbles on the surface of the metal | Lots of bubbles |
- Write the metals in order of reactivity, starting with the most reactive.
Priya and Carlos are investigating the reactivity of metals with different liquids.
Experiment : They add small pieces of the metals to water.
Experiment : They add small pieces of the metals to dilute hydrochloric acid.
Here are the results of the experiments:
| Metal |
Experiment : reaction with water |
Experiment : reaction with Hydrochloric acid. |
| A | Lots of bubbles | Lots of bubbles forming very quickly |
| B | No reaction | Some bubbles on the surface of metal |
| C | No reaction | No reaction |
| D | A few bubbles on the surface of the metal | Lots of bubbles |
- State which metal could be copper. Give a reason for your answer.
Priya and Carlos are investigating the reactivity of metals with different liquids.
Experiment : They add small pieces of the metals to water.
Experiment : They add small pieces of the metals to dilute hydrochloric acid.
Here are the results of the experiments:
| Metal |
Experiment : reaction with water |
Experiment : reaction with Hydrochloric acid. |
| A | Lots of bubbles | Lots of bubbles forming very quickly |
| B | No reaction | Some bubbles on the surface of metal |
| C | No reaction | No reaction |
| D | A few bubbles on the surface of the metal | Lots of bubbles |
- State which metal could be calcium. Give a reason for your answer.
