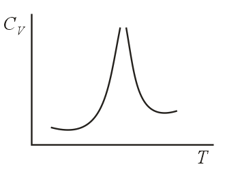
When diatomic gases are heated, their heat capacity exhibits a peak in the high-temperature region. Similar behavior is observed in multiatomic gases. What is the explanation for this?


Important Questions on Molecular Physics and Thermodynamics
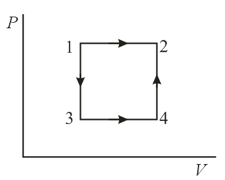
A gas is transferred from a state to a state by two processes: () first by an isochor and then by an isobar, and () first by an isobar and then by an isochor. Will the work done in both cases be the same, will the amount of heat required in the processes be the same, and will the increment of entropy in the processes be the same?
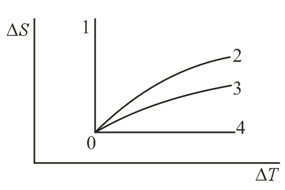
A gas is transferred from an initial state to other states and via different isoprocesses. Which curve representing the dependence of entropy on temperature corresponds to which process?
Suppose that the entropy grows linearly with temperature in a process. How does the heat capacity vary with temperature?
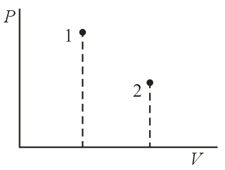
A gas is transferred from a state to a state in two ways: () directly by an isobar, and () first by the isochor ; then by the isobar , and, finally, by the isochor . Show, by direct calculation, that the entropy increment in both cases is the same.