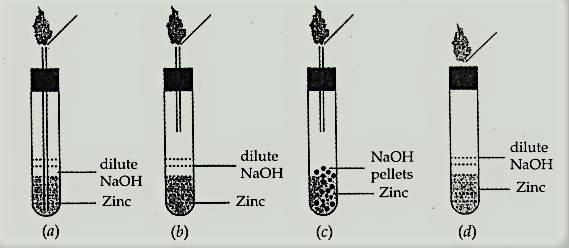
When dilute hydrochloric acid is added to granulated zinc placed in a test tube, the observation made is:

Important Questions on Multiple Choice Questions(MCQ's)
Ethanoic acid was added to sodium hydrogen carbonate solution and the gas evolved was tested with a burning splinter. The following observations were reported. The correct observation is:
Which one of the following set-ups is the most appropriate for the evolution of hydrogen gas and its identification?
Four students and noted the initial colour of the solutions in beakers I, II, III and IV. After inserting zinc rods in each solution and leaving it undisturbed for two hours, noted the colour of each solution again.
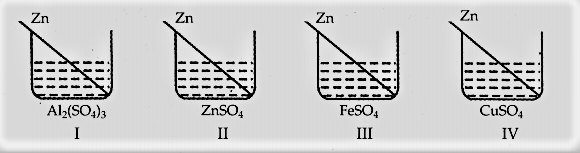
They recorded their observations in the form of a table given below:
| Student | Colour of the solution | ||||
| initial | colourless | colourless | Light green | Blue | |
| final | colourless | colourless | colourless | colourless | |
| initial | colourless | Light yellow | Light green | Blue | |
| final | colourless | colourless | Light green | colourless | |
| initial | colourless | colourless | Light green | Blue | |
| final | Light blue | colourless | colourless | Light blue | |
| initial | Light green | colourless | Light green | Blue | |
| final | colourless | colourless | Dark green | colourless |
Which student noted the colour change in all the four beakers correctly?
Four gas jars filled with sulphur dioxide gas were inverted into troughs of water by four students and the following observations and inference were reported:
(a) Water did not enter the gas jar and sulphur dioxide is insoluble in water.
(b) A small amount of water entered the gas jar slowly and sulphur dioxide is sparingly soluble in water.
(c) Water rushed into the gas jar and sulphur dioxide is highly soluble in water.
(d) Water did not enter the gas jar and sulphur dioxide is soluble in water.
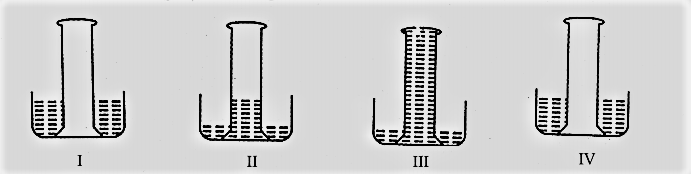
The correct set of observations and inference drawn is reported in:
