When dilute hydrochloric acid is added to iron filings,
Important Questions on Hydrogen
Complete and balance the following reactions:
When hydrogen is passed over a black solid compound ‘’, the products are a colourless liquid and a reddish brown metal ‘’ The substance ‘’ is divided into two parts and each part is placed in separate test tubes. To one part of the substance ‘’ dil. is added and to the other part dil. is added.
What would you observe when dil. nitric acid reacts with substance ‘’ ?
Thin strips of three different metals and are known to be magnesium, copper and iron.
Write down that you would observe in case when the metals are treated as follows:
When each metal is treated with dilute hydrochloric acid and warmed, if necessary.
The diagram below is an arrangement of apparatus for preparing and collecting hydrogen.
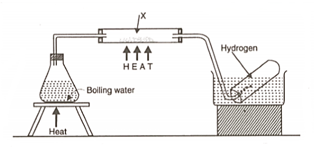
Write the equation for the reaction which takes place with steam and .
Complete and balance the following reactions:
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
Complete the following word equations:
Sodium hydroxide zinc Hydrogen .........
When hydrogen is passed over a black solid compound ‘’, the products are a colourless liquid and a reddish brown metal ‘’ The substance ‘’ is divided into two parts and each part is placed in separate test tubes. To one part of the substance ‘’ dil. is added and to the other part dil. is added.
Give two tests for the colourless liquid formed in the experiment.
The diagram below is an arrangement of apparatus for preparing and collecting hydrogen.
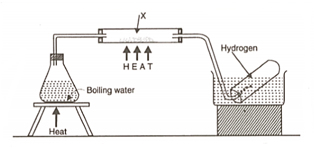
Name the substance .
Zinc is added to dilute sulphuric acid.

