EASY
Earn 100
When green light is incident on a certain metal surface, electrons are emitted but no electrons are emitted by yellow light. If red light is incident on the same metal surface
(a)More energetic electrons will be emitted
(b)Less energetic electrons will be emitted
(c)Emission of electrons will depend on the intensity of light
(d)No electrons will be emitted
50% studentsanswered this correctly
Important Questions on Dual Nature of Matter and Radiation
EASY
(Assume mass of electron and Charge of electron )
EASY
EASY
The photoelectric threshold for a certain metal surface is . If the metal surface is irradiated by a wavelength of , the kinetic energy of the emitted photoelectrons is
EASY
EASY
EASY
MEDIUM
EASY
EASY
Given (in )
EASY
EASY
EASY
( = Planck's constant, = speed of light)
EASY
EASY
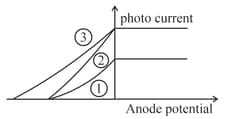
The following graph represents the variation of photo current with anode potential for a metal surface. Here and represents intensities and represents frequency for curves and respectively, then
EASY
EASY
EASY
EASY
EASY
EASY

