HARD
JEE Main/Advance
IMPORTANT
Earn 100
When semicarbazide reacts with a ketone (or aldehyde) to form semicarbazone. Only one nitrogen atom of semicarbazide acts as a nucleophile and attacks the carbonyl carbon of the ketone. The product of the reaction, consequently, is rather than . What factor account for the fact that two nitrogen atoms of semicarbazide are relatively non-nucleophilic?

Important Questions on Aldehydes, Ketones and Carboxylic Acids
HARD
JEE Main/Advance
IMPORTANT
Arrange the following compounds in decreasing orders of for hydrate formation.
(a)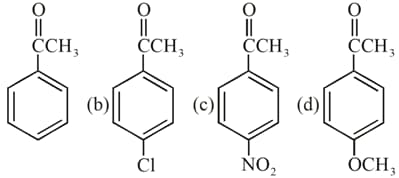
HARD
JEE Main/Advance
IMPORTANT
Which acetone when treated with excess of benzaldehyde in the presence of base results in the crossed condensation which adds two equivalents of benzaldehyde and expels two equivalents of water and forms Identify the structures of when reacts with How many stereo isomers are formed?
HARD
JEE Main/Advance
IMPORTANT
Predict the product for the following reactions.
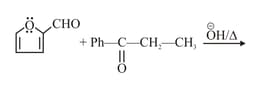
HARD
JEE Main/Advance
IMPORTANT
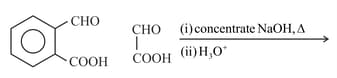
Product is:
HARD
JEE Main/Advance
IMPORTANT
An organic compound , in dry benzene in the presence of anhydrous gives compound . The compound on treatment with , followed by reaction with gives compound , which on reaction with hydrazine gives a cyclised compound Identify and . Explain the formation of from .
MEDIUM
JEE Main/Advance
IMPORTANT
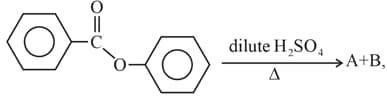 find and .
find and .
EASY
JEE Main/Advance
IMPORTANT
is.
HARD
JEE Main/Advance
IMPORTANT
In the given reaction the product is:
