When steam is passed through red hot iron, iron oxide and hydrogen gas is formed. The balanced equation for the reaction is shown below.
What is true for the balanced chemical equation shown above?

Important Questions on Chemical Reactions and Equations
The equation given below shows the reaction for cellular respiration. Cellular respiration is a chemical process by which cells convert glucose to energy.
In the above reaction, which substance is oxidised?
The equation given below shows the reaction for cellular respiration. Cellular respiration is a chemical process by which cells convert glucose to energy.
Carbon dioxide and water are the two new substances formed during cellular respiration. What are they known as?
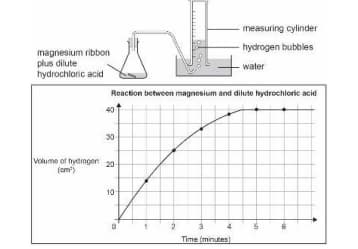
A piece of magnesium ribbon is added to a flask containing dilute hydrochloric acid.
Hydrogen gas is formed which is collected in the measuring cylinder.
The line on the graph indicates the rate of chemical reaction occurring in the flask.
The amount of hydrogen formed with time is plotted on a graph.
 .
.
At what time is the reaction rate the fastest in the flask?
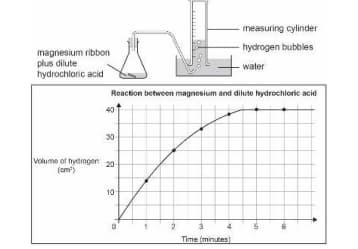
A piece of magnesium ribbon is added to a flask containing dilute hydrochloric acid.
Hydrogen gas is formed which is collected in the measuring cylinder.
The line on the graph indicates the rate of chemical reaction occurring in the flask.
The amount of hydrogen formed with time is plotted on a graph.
 .
.
The reaction is repeated with magnesium powder in place of magnesium ribbon under the same conditions. Will the reaction rate increase or decrease?
Explain your answer with reference to the volume of hydrogen formed in the flask at 2 minutes.
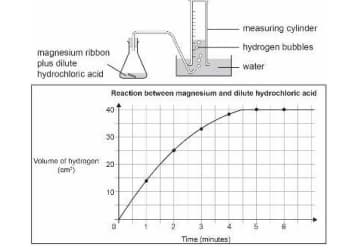
A piece of magnesium ribbon is added to a flask containing dilute hydrochloric acid.
Hydrogen gas is formed which is collected in the measuring cylinder.
The line on the graph indicates the rate of chemical reaction occurring in the flask.
The amount of hydrogen formed with time is plotted on a graph.
 .
.
Which of these could increase the rate of reaction in the flask?
Magnesium reacts with hydrochloric acid to form magnesium chloride and hydrogen gas.
Write a balanced chemical equation to show the reaction.
