When the frequency of the incident radiation on a metallic plate is doubled, KE of the photolectrons will be
Important Questions on Structure of Atom
When light of wavelength falls on a metal of threshold energy , the de-Broglie wavelength of emitted electrons is ________ . (Round off to the Nearest Integer).
[Use :
What is the work function of the metal if the light of wavelength generates photoelectrons of velocity from it?
(Mass of electron , velocity of light , Planck's constant , Charge of electron )
The minimum energy that must be possessed by photons in order to produce the photoelectric effect with platinum metal is:
[Given: The threshold frequency of platinum is and ]
With regard to photoelectric effect, identify the CORRECT statement among the following:
If is the momentum of the fastest electron ejected from a metal surface after the irradiation of light having wavelength then for momentum of the photoelectron, the wavelength of the light should be:
(Assume kinetic energy of ejected photoelectron to be very high in comparison to work function)
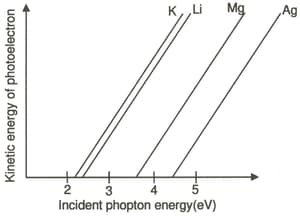
The photoelectric behaviour of and metals is shown in the plot below. If light of wavelength is incident on each of these metals, which of them will emit photoelectrons? [Planck's contant ; velocity of light ]
[Given : , Mass of electron ], Give an answer to the nearest integer value.
[Given : , and ]

