MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
When two moles of hydrogen atoms join together to form a mole of hydrogen molecules in a closed rigid vessel with diathermic walls.
(a)
(b) negative
(c) positive
(d) negative
60% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
In figure, and are two adiabatic curves for two different gases. Then and corresponds to:
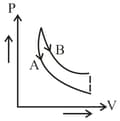
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
This phase transition is carried out at constant temp and pressure then work done during the process:
MEDIUM
JEE Main/Advance
IMPORTANT
