When we add dilute sulphuric acid to salt, brisk effervescence is observed then the possible anion is carbonate.
Important Questions on Inorganic Qualitative Analysis
side products
side products
side products
The sum of the total number of atoms in one molecule each of and is ________
Distinguish between the following pair of compounds using a reagent as a chemical test:
- Magnesium chloride and magnesium nitrate solution.
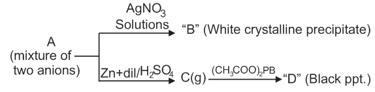
Then A may have:
The _____ gas is released when hydrochloric acid is added to the test compound. It indicates the presence of carbonate ions.
A dilute acid is used to detect carbonate ions, CO32- Bubbles are released when an acid is added to the test compound, usually diluted hydrochloric acid. Carbon dioxide causes the bubbles using Limewater and the gas is carbon dioxide.
To an acid solution of an anion a few drops of KMnO4 solution are added. Which of the following, if present will not decolourise the KMnO4 solution
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
Note: Question details are changed from that of the book.
Select the correct option(s) for the white precipitate shown in the above reactions.

