MEDIUM
9th CBSE
IMPORTANT
Earn 100
When zinc metal reacts with dilute sulphuric acid, a gas is evolved. Which one is a correct statement about the nature of this gas?
(a)colourless with suffocating odour
(b)reddish brown and odourless
(c)colourless and sweet smelling
(d)colourless and odourless
46.15% studentsanswered this correctly

Important Questions on Multiple Choice Questions (MCQs) (Based on Practical Skills in Science)
MEDIUM
9th CBSE
IMPORTANT
The reaction between iron and copper sulphate solution represents which type of reaction?
EASY
9th CBSE
IMPORTANT
When a burning magnesium ribbon is introduced in a gas jar containing oxygen, it will burn with:
MEDIUM
9th CBSE
IMPORTANT
A student heats calculated amounts of iron filings and sulphur powder together in a boiling tube. He will obtain
MEDIUM
9th CBSE
IMPORTANT
Which of the following mixture can be separated completely by the process of sublimation?
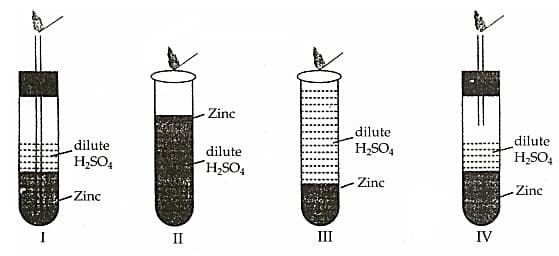
HARD
9th CBSE
IMPORTANT
Four set-ups as given here were arranged to identify the gas evolved when dilute sulphuric acid was added to zinc granules. The most appropriate set up is
EASY
9th CBSE
IMPORTANT
An iron nail was dropped into copper sulphate solution. After some time, the colour of the solution changed from
MEDIUM
9th CBSE
IMPORTANT
A student was asked by her science teacher to prepare a true solution. She dissolved the solute in water but forgot to record its name. What may be the correct name of the solute?
MEDIUM
9th CBSE
IMPORTANT
A student placed a clean iron nail in blue coloured copper sulphate solution for a considerable time. He observes that :
