EASY
8th Maharashtra Board
IMPORTANT
Earn 100
Where are electrons revolving around the nucleus placed?

Important Questions on Inside the Atom
HARD
8th Maharashtra Board
IMPORTANT
EASY
8th Maharashtra Board
IMPORTANT
EASY
8th Maharashtra Board
IMPORTANT
EASY
8th Maharashtra Board
IMPORTANT
EASY
8th Maharashtra Board
IMPORTANT
HARD
8th Maharashtra Board
IMPORTANT
Complete the table:
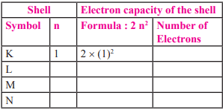
MEDIUM
8th Maharashtra Board
IMPORTANT
Use the following molecular formulae to determine the valencies of .
Molecular formulae of
EASY
8th Maharashtra Board
IMPORTANT
