Which British law did the Salt Satyagraha aim to break?
Important Questions on The d- and f-Block Elements
characteristic of a copper wire of length and area of cross-section is shown in figure. The slope of the curve becomes
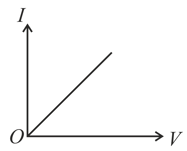
Write the relationship between electrical resistance and electrical resistivity for a metallic conductor of cylindrical shape. Hence, derive the SI unit of electrical resistivity.
Given below are two statement : one is labelled as Assertion A and the other is labelled as Reason R :
Assertion A : Size of ion is less than ion.
Reason R : The above is a consequence of the lanthanide contraction.
In the light of the above statements, choose the correct answer from the options given below :
Find the resistivity of the material of a metallic conductor of length and area of cross-section . The resistance of the conductor is .
Give reason for actinides show irregularities in their electronic configuration.
Give reason: Actinoid contraction is greater from element to element than lanthanoid contraction.

