MEDIUM
Earn 100
Which constitutional amendment extended the reservation of seats for Scheduled Castes and Scheduled Tribes in the House of the People and the Legislative Assemblies of the States for another 10 years?
(a)104th Amendment
(b)77th Amendment
(c)95th Amendment
(d)91st Amendment
50% studentsanswered this correctly
Important Questions on Linear Inequalities
EASY
MEDIUM
MEDIUM
EASY
EASY
The following Venn diagram indicates
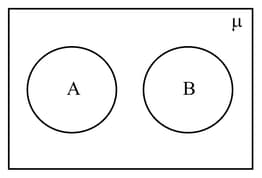
HARD
EASY
EASY
EASY
EASY
EASY
EASY
MEDIUM
EASY
EASY
EASY
EASY
MEDIUM
MEDIUM

