MEDIUM
Earn 100
Which graph indicate the trend of valency in a group?
(a)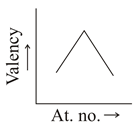

(b)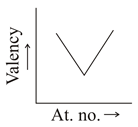

(c)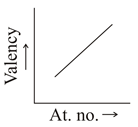

(d)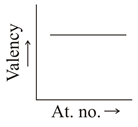

50% studentsanswered this correctly
Important Questions on Classification of Elements and Periodicity in Properties
MEDIUM
The first ionization energy of magnesium is smaller, as compared to that of elements and , but higher than that of . The elements and , respectively, are :
EASY
Which of the following pairs exhibit a diagonal relationship?
EASY
and show similarities in properties due to the diagonal relationship except in the property given below. What is ?
MEDIUM
Given below are the oxides :
and Number of amphoteric oxides is :
EASY
In general, the property (magnitudes only) that shows an opposite trend in comparison to other properties across a period is :
EASY
The characteristics of elements and with atomic numbers, respectively, and are:
EASY
Identify the correct statement from the following.
EASY
Total number of acidic oxides among and is _____ .
MEDIUM
Three elements and are in the period of the periodic table. The oxides of and , respectively, are basic, amphoteric and acidic. The correct order of the atomic numbers of and is:
EASY
Match the following :
| Oxide | Nature |
| (a) | (i) Basic |
| (b) | (ii) Neutral |
| (c) | (iii) Acidic |
| (d) | (iv) Amphoteric |
Which of the following is correct option?
MEDIUM
The total number of acidic oxides from the following list is:
MEDIUM
The acidic, basic and amphoteric oxides, respectively, are
EASY
The correct decreasing order for metallic character is
EASY
The set of elements that differ in mutual relationship from those of the other sets is:
EASY
Match List I with List II
| List I | List II | ||
| Elements | Colour imparted to the flame | ||
| A | I | Brick Red | |
| B | II | Violet | |
| C | III | Apple Green | |
| D | IV | Crimson Red | |
Choose the correct answer from the options given below:
EASY
Identify the element that forms amphoteric oxide:
EASY
In the following reaction, the total number of oxygen atoms in and is______
EASY
Which among the following oxides is the most basic?
HARD
has a smaller first ionization enthalpy than . Consider the following statement:
(I) it is easier to remove electron than electron
(II) electron of is more shielded from the nucleus by the inner core of electrons than the electrons of
(III) electron has more penetration power than electron
(IV) atomic radius of is more than
(atomic number )
The correct statements are.
MEDIUM
In the following sets of reactants which two sets best exhibit the amphoteric character of Al2O3 . xH2O ?

