EASY
Earn 100
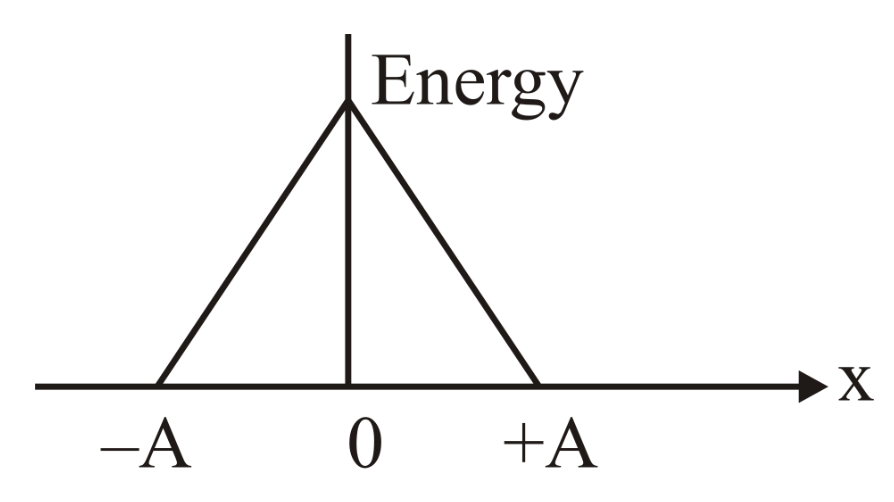
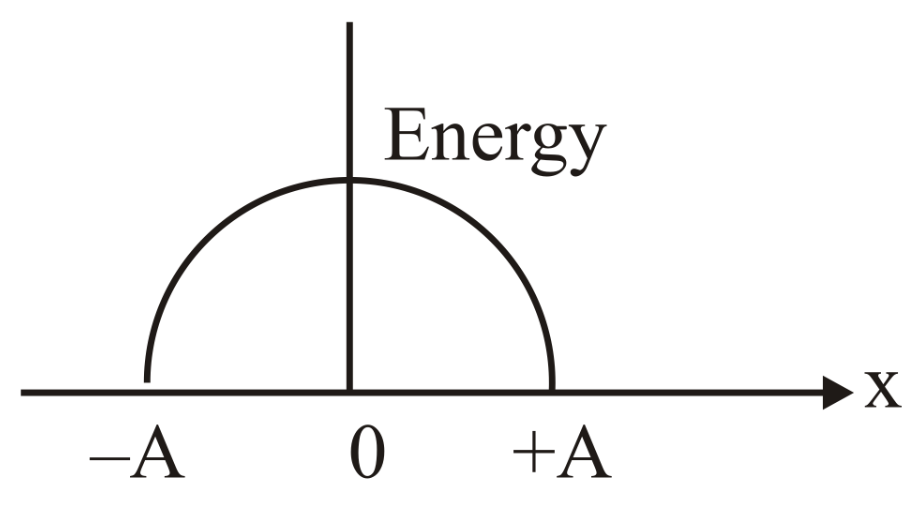
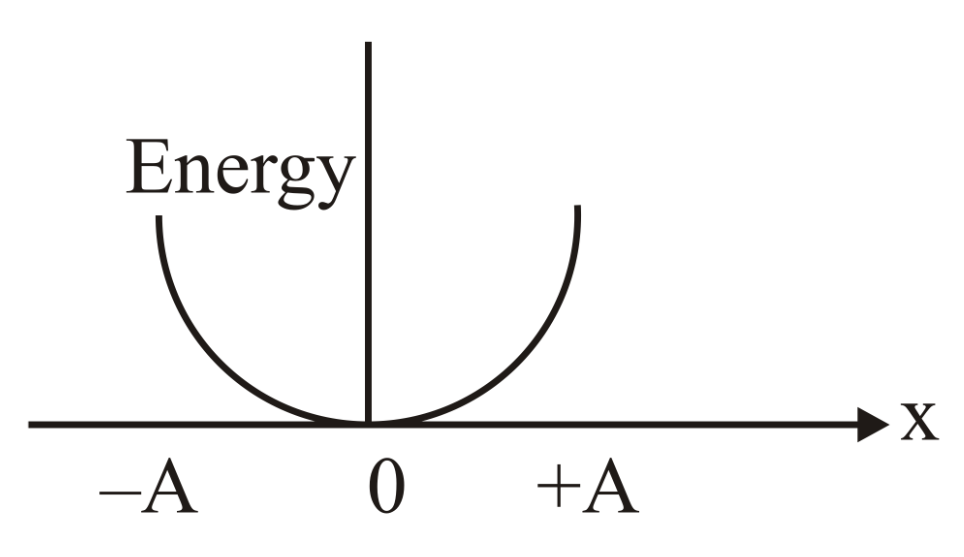
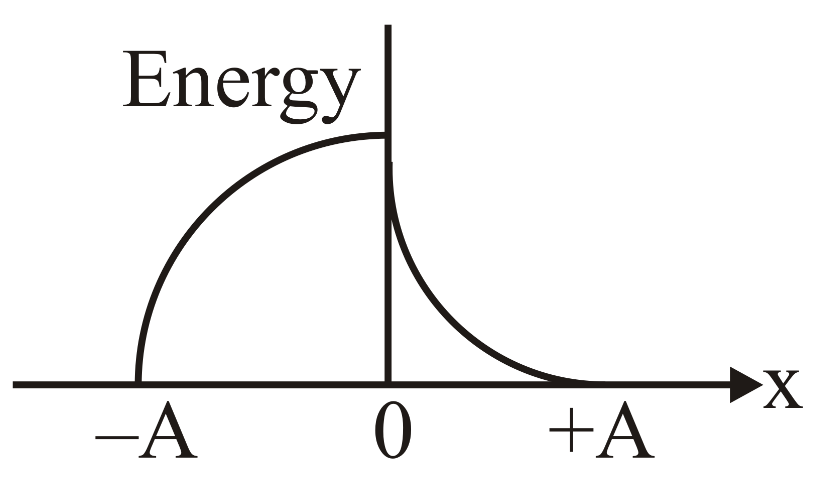
Which graph represents the difference between total energy and potential energy of a particle executing vs its distance from mean position?
(a)
(b)
(c)
(d)
65.91% studentsanswered this correctly
Important Questions on Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).
HARD
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
HARD
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
EASY
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
MEDIUM
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
MEDIUM
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
MEDIUM
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
EASY
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
EASY
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
HARD
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
EASY
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
MEDIUM
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
A mass of is connected to a spring. The potential energy curve of the simple harmonic motion executed by the system is shown in the figure. A simple pendulum of length has the same period of oscillation as the spring system. What is the value of acceleration due to gravity on the planet where these experiments are performed ?
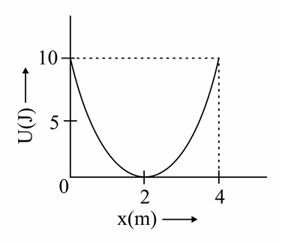
MEDIUM
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
MEDIUM
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
HARD
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
MEDIUM
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
(a) Potential energy is always equal to its
(b) Average potential and kinetic energy over any given time interval are always equal.
(c) Sum of the kinetic and potential energy at any point of time is constant.
(d) Average in one time period is equal to average potential energy in one time period.
Choose the most appropriate option from the options given below :
HARD
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
HARD
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
MEDIUM
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
EASY
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)
MEDIUM
Physical Sciences>Energy>Plan and conduct an investigation to provide evidence that the transfer of thermal energy when two components of different temperature are combined within a closed system results in a more uniform energy distribution among the components in the system (second law of thermodynamics).>Conservation of Energy and Energy Transfer - Energy cannot be created or destroyed, but it can be transported from one place to another and transferred between systems.Uncontrolled systems always evolve toward more stable states—that is, toward more uniform energy distribution (e.g., water flows downhill, objects hotter than their surrounding environment cool down)

