HARD
Diploma
IMPORTANT
Earn 100
Which indicator would be the most appropriate for titrating aqueous ethylamine, , with nitric acid, ?
(a)Bromophenol blue
(b)Bromothymol blue
(c)Phenol red
(d)Thymolphthalein
50% studentsanswered this correctly

Important Questions on Acids and Bases (AHL)
HARD
Diploma
IMPORTANT
The graph below shows the titration curve of of of hydrochloric acid with sodium hydroxide, of concentration. The indicator methyl orange was used to determine the equivalence point. Methyl orange has a pH range of .
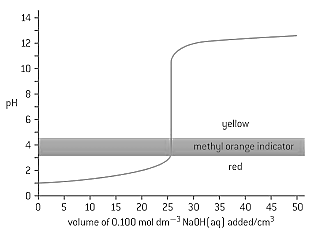
If the hydrochloric acid was replaced by ethanoic acid of the same volume and concentration, which property of the titration would remain the same?
