EASY
JEE Main
IMPORTANT
Earn 100
Which intermolecular force is most responsible in allowing xenon gas to liquefy?
(a)London forces
(b)Ion-dipole
(c)Ionic
(d)Dipole-dipole
71.43% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
MEDIUM
JEE Main
IMPORTANT
The temperature at which oxygen molecules have the same root mean square speed as helium atoms have at 300 K is : (Atomic masses : He = 4 u, O = 16 u)
HARD
JEE Main
IMPORTANT
Van der Waal's equation for a gas is stated as,
.
This equation reduces to the perfect gas equation, When,
HARD
JEE Main
IMPORTANT
A graph of vapour pressure and temperature for three different liquids and is shown below:
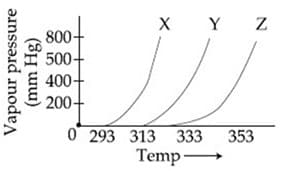
The following inferences are made:
has higher intermolecular interactions compared to
has lower intermolecular interactions compared to
has lower intermolecular interactions compared to
The correct inferences is/are:
EASY
JEE Main
IMPORTANT
The predominant intermolecular forces present in ethyl acetate, a liquid, are:
EASY
JEE Main
IMPORTANT
Among the following, the incorrect statement is:
MEDIUM
JEE Main
IMPORTANT
Identify the correct labels of and in the following graph from the options given below:
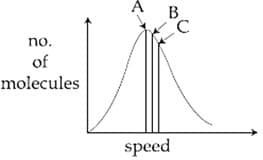
Root mean square speed most proable speed Average speed
MEDIUM
JEE Main
IMPORTANT
At the vapour pressure of is and that of acetone is A solution of in acetone has a total vapour pressure of . The false statement amongst the following is:
EASY
JEE Main
IMPORTANT
The relative strength of the interionic/ intermolecular forces in a decreasing order is:
