MEDIUM
JEE Main
IMPORTANT
Earn 100
Which is larger, an ion with an electron in an orbit with or a ion with an electron in an orbit with ?
(a)
(b)
(c)Both equal
(d)None of these
100% studentsanswered this correctly

Important Questions on Structure of Atom
HARD
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
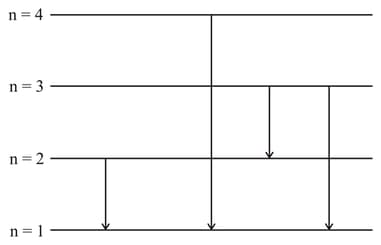
Suppose that a hypothetical atom gives a red, green, blue & violet line spectrum. Which jump according to the figure would give off the red spectral line?
MEDIUM
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
