MEDIUM
12th ICSE
IMPORTANT
Earn 100
Which is not affected by temperature?
(a)Normality
(b)Formality
(c)Molarity
(d)Molality
50% studentsanswered this correctly

Important Questions on Solutions
MEDIUM
12th ICSE
IMPORTANT
MEDIUM
12th ICSE
IMPORTANT
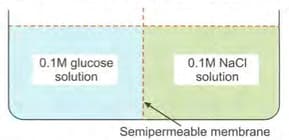
Consider the following figure and choose the correct option:
MEDIUM
12th ICSE
IMPORTANT
MEDIUM
12th ICSE
IMPORTANT
HARD
12th ICSE
IMPORTANT
MEDIUM
12th ICSE
IMPORTANT
HARD
12th ICSE
IMPORTANT
MEDIUM
12th ICSE
IMPORTANT
