HARD
Earn 100
Which is the most abundant noble gas?
(a)Argon
(b)Helium
(c)Neon
(d)Krypton
25% studentsanswered this correctly
Important Questions on The p-Block Elements
MEDIUM
MEDIUM
Give a reason for the following:
Inert gases do not form ions.
EASY
EASY
EASY
MEDIUM
| Column I | Column II | ||
| (a) | (i) | Distorted octahedral | |
| (b) | (ii) | Square planar | |
| (c) | (iii) | Pyramidal | |
| (d) | (iv) | Square pyramidal |
HARD
MEDIUM
Among the following molecules,
(i)
(ii)
(iii)
those having same number of lone pairs on are:
EASY
MEDIUM
EASY
EASY
HARD
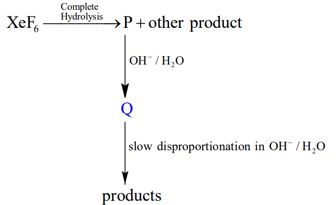
Under ambient conditions, the total number of gases released as products in the final step of the reaction scheme shown below is
EASY
MEDIUM
MEDIUM
Mention the hybridisation in the following molecule or ion :
HARD
HARD
The following table represents the elements and the atomic number. With reference to this, answer the following using only the alphabets given in the table.
| Element | Atomic number |
Which element has an electron affinity zero?
EASY
EASY

