EASY
Earn 100
Which is the weakest among the following type of bond ?
(a)Ionic bond
(b)Covalent bond
(c)Metallic bond
(d)Hydrogen bond
100% studentsanswered this correctly
Important Questions on States of Matter: Gaseous and Liquid States
EASY
[ is the distance between the polar molecules]
EASY
Increasing order of boiling points in the following compounds is:
EASY
MEDIUM
EASY
HARD
MEDIUM
MEDIUM
The combination of plots which does not represent isothermal expansion of an ideal gas is
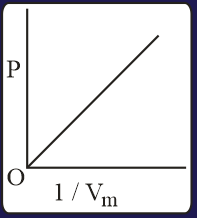
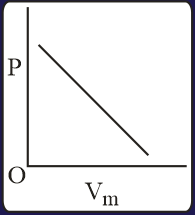
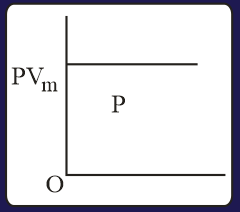
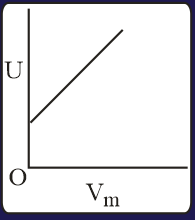
EASY
EASY
EASY
(Latent heat of ice is and )
EASY
MEDIUM
MEDIUM
In which of the following solid substance dispersion forces exist?
HARD
HARD
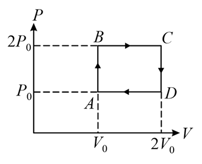
The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
HARD
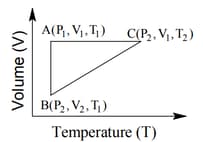
The correct option(s) is (are)
MEDIUM
Match the type of interaction in column with the distance dependence of their interaction energy in column
| A | B |
| (i) ion - ion | (a) |
| (ii) Dipole - dipole | (b) |
| (iii) London dispersion | (c) |
| (d) |
EASY
EASY

