MEDIUM
Earn 100
Which of the following alkane can be obtained in good yield by the Wurtz reaction?
(a)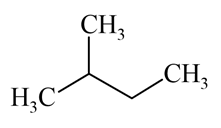

(b)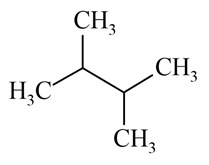

(c)
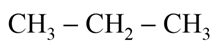
(d)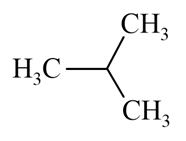

56.6% studentsanswered this correctly
Important Questions on Hydrocarbons
EASY
Wurtz reaction
Reduction of alkyl halides with zinc and dil.
Kolbe's electrolysis
Catalytic hydrogenation of alkenes
EASY
Which of the following reactions is used for producing symmetrical alkane with double carbon atoms?
MEDIUM
Assertion (A): Sodium acetate on Kolbe's electrolysis gives ethane.
Reason (R): Methyl free radical is formed at cathode.
EASY
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
Which hydrogen in compound is easily replaceable during bromination reaction in presence of light ?
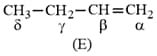
MEDIUM
MEDIUM

