EASY
Earn 100
Which of the following are characteristics of a real gas?
(a)The molecules attract each other
(b)It obeys the ideal gas law at low temperature and high pressure
(c)The mass of molecule is negligible
(d)It shows deviation from the ideal gas law
50% studentsanswered this correctly
Important Questions on States of Matter : Gases and Liquids
EASY
| Gas | ||
|---|---|---|
| A | ||
| B | ||
| C | ||
| D |
and are vander Waals constants. The correct statement about the gases is:
EASY
EASY
HARD
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
HARD
.
This equation reduces to the perfect gas equation, When,
EASY
MEDIUM
The variation of the compressibility factorwith pressure for some gases, are shown in the figure below. Identify the gases and .
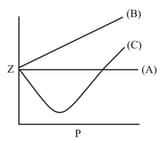
MEDIUM
HARD
In thermodynamics, the work done is given by . For a system undergoing a particular process, the work done is,
. This equation is applicable to a
HARD
MEDIUM
EASY
For a van der Waals' gas, the term represents some
HARD
HARD
EASY

