HARD
Earn 100
Which of the following best describes the electronic structure of the cation formed by protonation of propyne?
(a)It is an allylic carbocation mainly stabilized by resonance with the sp2 hybridized C
(b)It is vinylic carbocation stabilized by resonance and hyperconjugation
(c)It is a &nbps;carbocation stabilized by hyperconjugation and resonance with the sp3 hybridized C
(d)It is vinylic carbocation stabilized mainly by hyperconjugation with the sp-hybridized cationic carbon
50% studentsanswered this correctly
Important Questions on Hydrocarbons
MEDIUM
In the compound,
The most acidic hydrogen atom is
MEDIUM
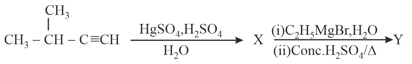
HARD
MEDIUM
The major product in the following reactions is
EASY
Which among the following depicts the correct order of acidity?
MEDIUM
MEDIUM
The correct sequence of reagents for the following conversion will be
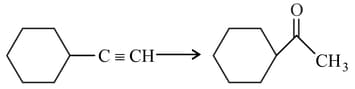
HARD
EASY
MEDIUM
The major product of the following reaction is
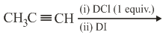
EASY
MEDIUM
In the reaction
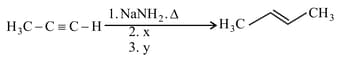
and , respectively, are
MEDIUM
MEDIUM
HARD
EASY
Match List with List
| List (Chemicals) |
List (Use / Preparation / Constituent) |
||
| (a) | Alcoholic potassium hydroxide | (i) | Electrodes in batteries |
| (b) | (ii) | Obtained by addition reaction | |
| (c) | (Benzene hexachloride) | (iii) | Used for elimination reaction |
| (d) | Polyacetylene | (iv) | Lindlar's catalyst |
Choose the most appropriate match
EASY
HARD
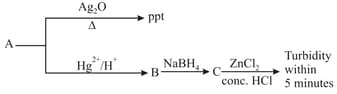
‘A’ is:
EASY
MEDIUM

