MEDIUM
Earn 100
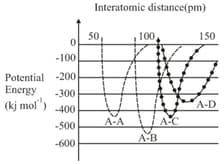
Which of the following bonds required the largest amount of bond energy to dissociate the atom concerned?
(a)
(b)
(c)
(d)
66.67% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
EASY
MEDIUM
EASY
How many ions of the following have a bond order of
MEDIUM
MEDIUM
HARD
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
Find out the correct order of ionic character in the following molecules
MEDIUM
The bond lengths (in of and are:
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
EASY

