MEDIUM
JEE Main
IMPORTANT
Earn 100
Which of the following cannot determine the state of a thermodynamic system?
(a)Pressure and volume.
(b)Volume and temperature.
(c)Temperature and pressure.
(d)Any one of pressure, volume or temperature.
72.81% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
If ideal diatomic gas follows the process as shown in graph where is temperature in and is volume , then molar heat capacity for this process will be [in terms of gas constant ],
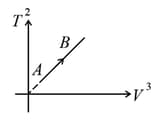
MEDIUM
JEE Main
IMPORTANT
Air in a cylinder is suddenly compressed by a piston, which is then maintained at the same position. With the passage of time,
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
Ideal gas is taken through a process as shown in figure
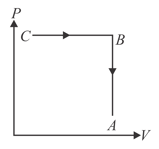
MEDIUM
JEE Main
IMPORTANT
