MEDIUM
Earn 100
Which of the following carbonates do not give metal oxide on heating?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Inorganic Qualitative Analysis
HARD
EASY
MEDIUM
Match List I with List II.
| List-I (Anion) |
List-II (gas evolved on reaction with dil. ) |
||
| (A) | (I) |
Colourless gas which turns lead acetate paper black. |
|
| (B) | (II) |
Colourless gas which turns acidified potassium dichromate solution green. |
|
| (C) | (III) | Brown fumes which turns acidified KI solution containing starch blue. | |
| (D) | (IV) | Colourless gas evolved with brisk effervescence, which turns lime water milky. |
Choose the correct answer from the options given below
EASY
The blue colour produced when a starch solution is added to a solution containing traces of is due to
(I) formation of
(II) formation of an inclusion complex
(III) Oxidation of starch
(IV) Oxidation of
EASY
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
HARD
EASY
MEDIUM
HARD
MEDIUM
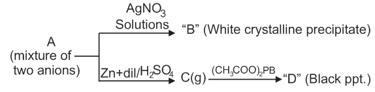
Then A may have:
MEDIUM
EASY
EASY
EASY
MEDIUM

