MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Which of the following complexes can exist as enantiomers? Draw their structures

Important Questions on Coordination Compounds
HARD
JEE Main/Advance
IMPORTANT
Draw the structures of the following metal carbonyls
MEDIUM
JEE Main/Advance
IMPORTANT
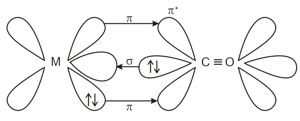
The figure represents the synergic bonding interaction in metal carbonyl complex. On the basis of this explain the following:
The strength of Metal-ligand bond.
The bond order of in carbonyl complex as compared to bond order in carbon monoxide.
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
