EASY
Earn 100
Which of the following covalent solid is the conductor of electricity?
(a)
(b)
(c)Diamond
(d)Graphite
50% studentsanswered this correctly
Important Questions on Solid State
EASY
MEDIUM
Match List - I with List - II:
| List-I (Property) | List-II (Example) | ||
| (a) | Diamagnetism | (i) | |
| (b) | Ferrimagnetism | (ii) | |
| (c) | Paramagnetism | (iii) | |
| (d) | Antiferromagnetism | (iv) | |
Choose the most appropriate answer from the options given below:
EASY
HARD
Remove all the anions , except the central one.
Replace all the face centered cations , by anions .
Remove all the corner cations .
Replace the central anion , with cation .
The value of in is _____.
MEDIUM
HARD
HARD
Match the following:
| doped with | n-type semiconductor | ||
| doped with | Schottky defect | ||
| p-type semiconductor | |||
| Frenkel defect |
EASY
type of semi-conductor material
amount of doping
temperature
Which one of the following is correct?
MEDIUM
EASY
EASY
EASY
MEDIUM
Copper shows electrical conductance in solid as well as molten state whereas copper chloride shows electrical conductance only in molten state. Give reason.
MEDIUM
HARD
The cubic unit cell structure of a compound containing cation and anion is shown below. When compared to the anion, the cation has smaller ionic radius. Choose the correct statement(s).
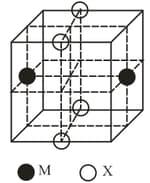
MEDIUM
EASY
EASY

