MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Which of the following curve does not represent Boyle's law?
(a)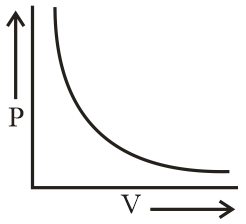

(b)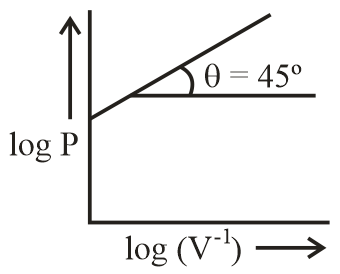

(c)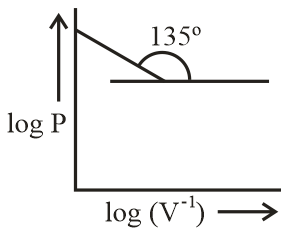

(d)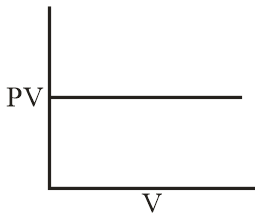

50% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
EASY
JEE Main/Advance
IMPORTANT
The density of liquid gallium at is . Because of its wide liquid range ( to) gallium could be used as a barometer fluid at high temperature. What height (in ) of gallium will be supported on a day when the mercury barometer reads ? (The density of mercury is ).
MEDIUM
JEE Main/Advance
IMPORTANT
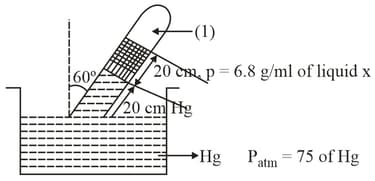
Pressure of the gas in column is:
EASY
JEE Main/Advance
IMPORTANT
A gas is heated from to at pressure. If the initial volume of the gas is , its final volume would be
MEDIUM
JEE Main/Advance
IMPORTANT
If the pressure of a gas contained in a closed vessel is increased by when heated by , the initial temperature must be:
MEDIUM
JEE Main/Advance
IMPORTANT
A thin balloon filled with air at has a volume of litres. If on placing it in a cooled room, its volume becomes litres, the temperature of the room is :
EASY
JEE Main/Advance
IMPORTANT
If a mixture containing moles of hydrogen and mole of nitrogen is converted completely into ammonia, the ratio of the initial and final volume under the same temperature and pressure would be
EASY
JEE Main/Advance
IMPORTANT
at contained in a flask was replaced by under identical conditions of pressure, temperature and volume. Then, the weight of will be ____ of .
EASY
JEE Main/Advance
IMPORTANT
Under what conditions, will a pure sample of an ideal gas not only exhibit a pressure of but also a concentration of
