EASY
Earn 100
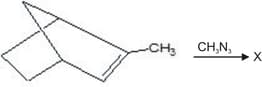
Which of the following cycloalkane gives open chain compound, when reacted with bromine?
(a)Cyclopropane
(b)Cyclopentane
(c)Cyclohexane
(d)Cyclooctane
50% studentsanswered this correctly
Important Questions on Hydrocarbons
EASY
MEDIUM
EASY
EASY
EASY
MEDIUM
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
EASY
EASY
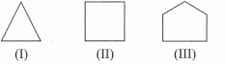
The correct order of reactivity of towards addition reactions is:
EASY
EASY
MEDIUM
MEDIUM
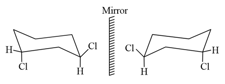
Efforts to resolve this compound fail. why?
MEDIUM
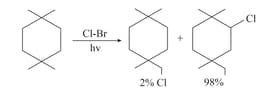
A compound Gives only two monochloro product on chlorination (excluding stereoisomers) P is
EASY
MEDIUM
EASY