HARD
JEE Main
IMPORTANT
Earn 100
Which of the following figure represents the variation of the particle momentum and the associated de Broglie wavelength?
(a)
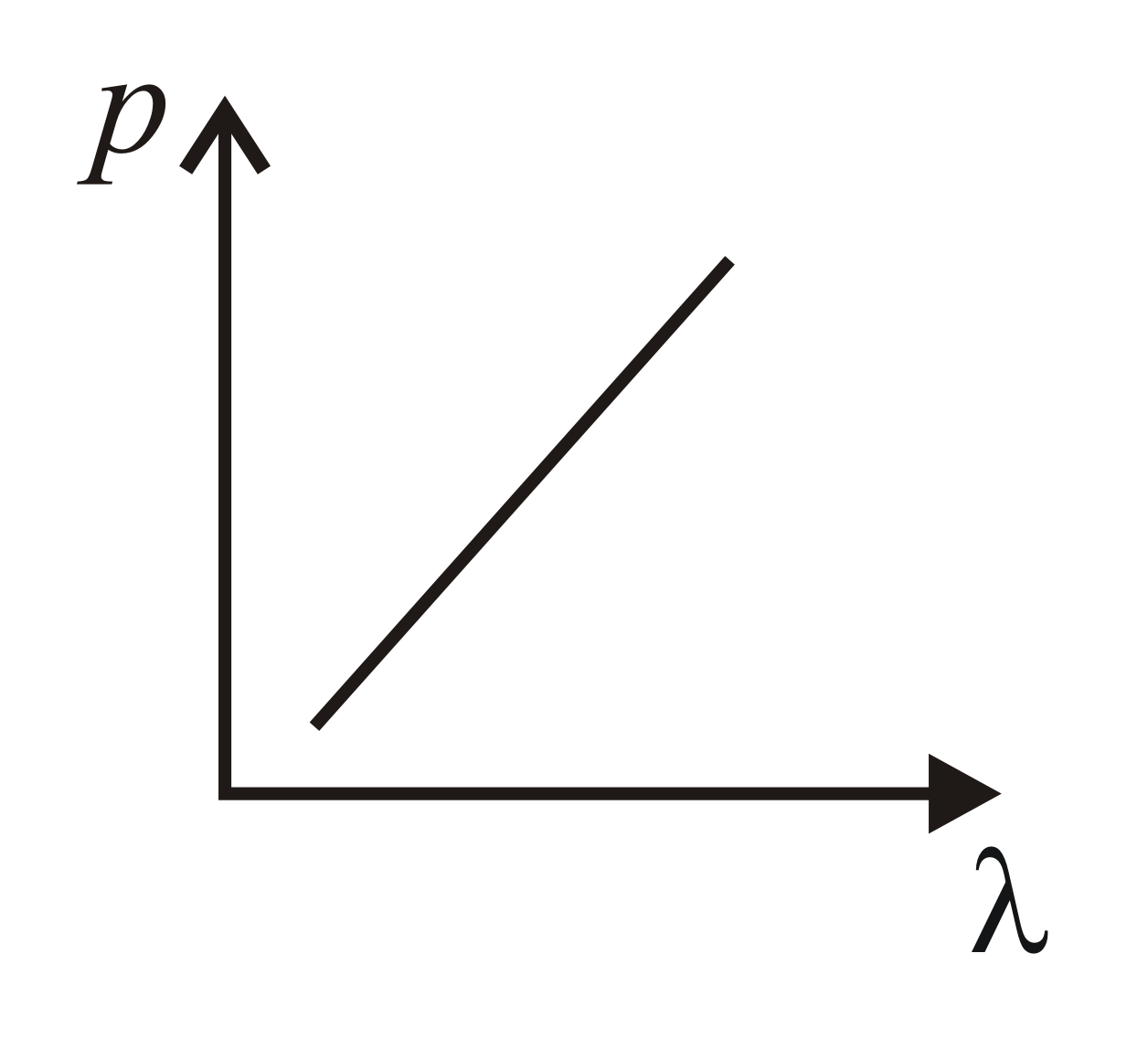
(b)
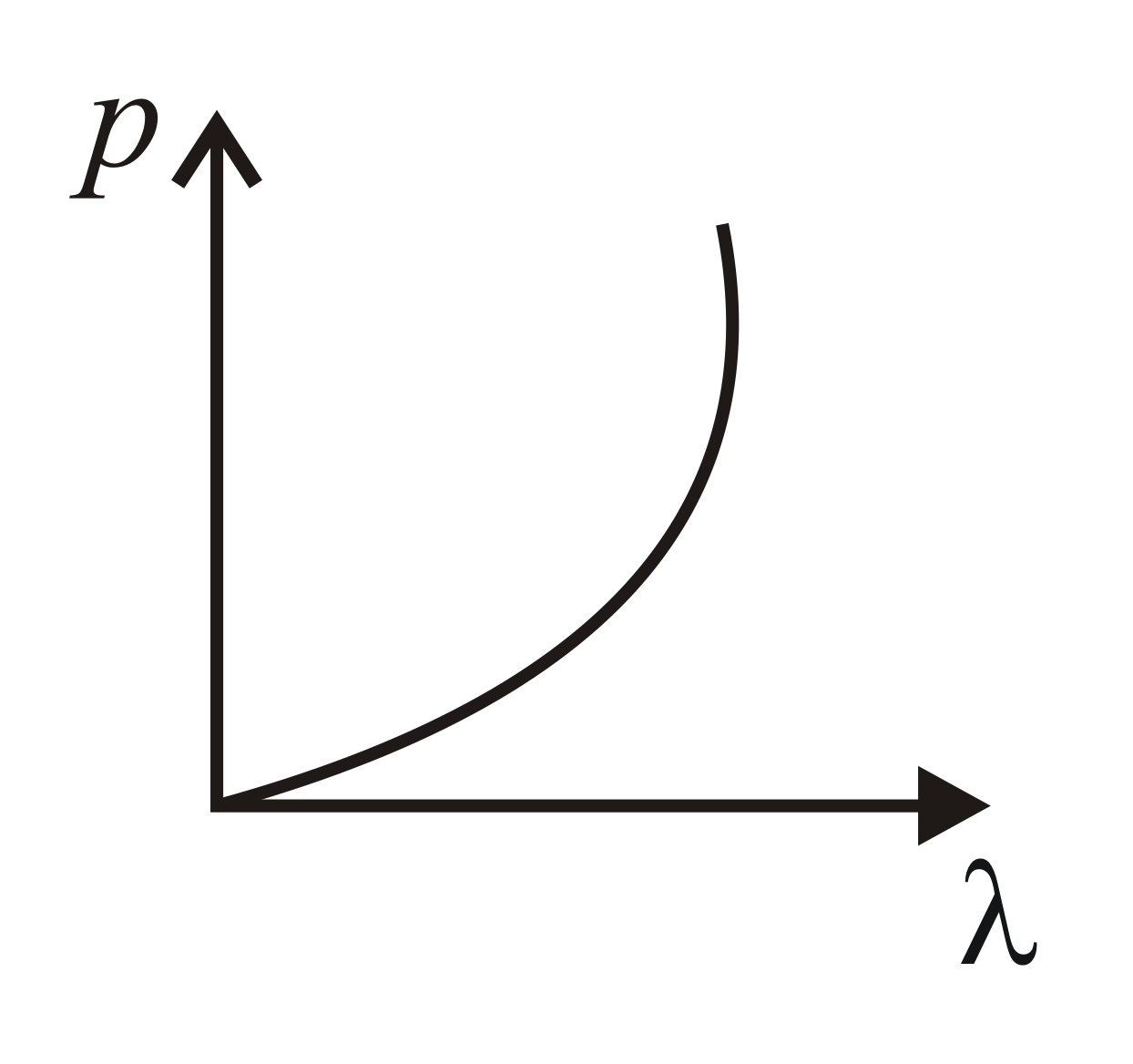
(c)
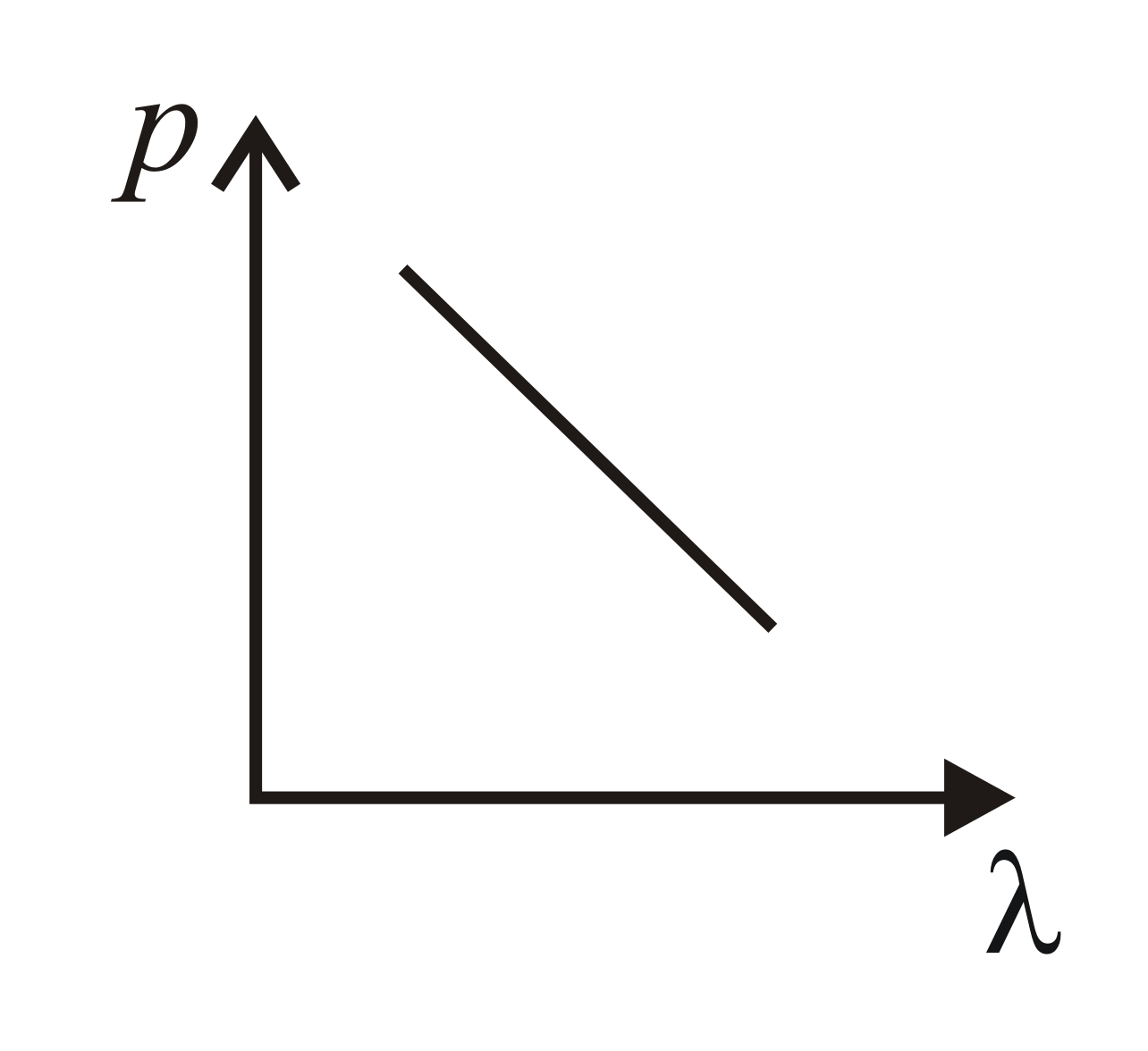
(d)
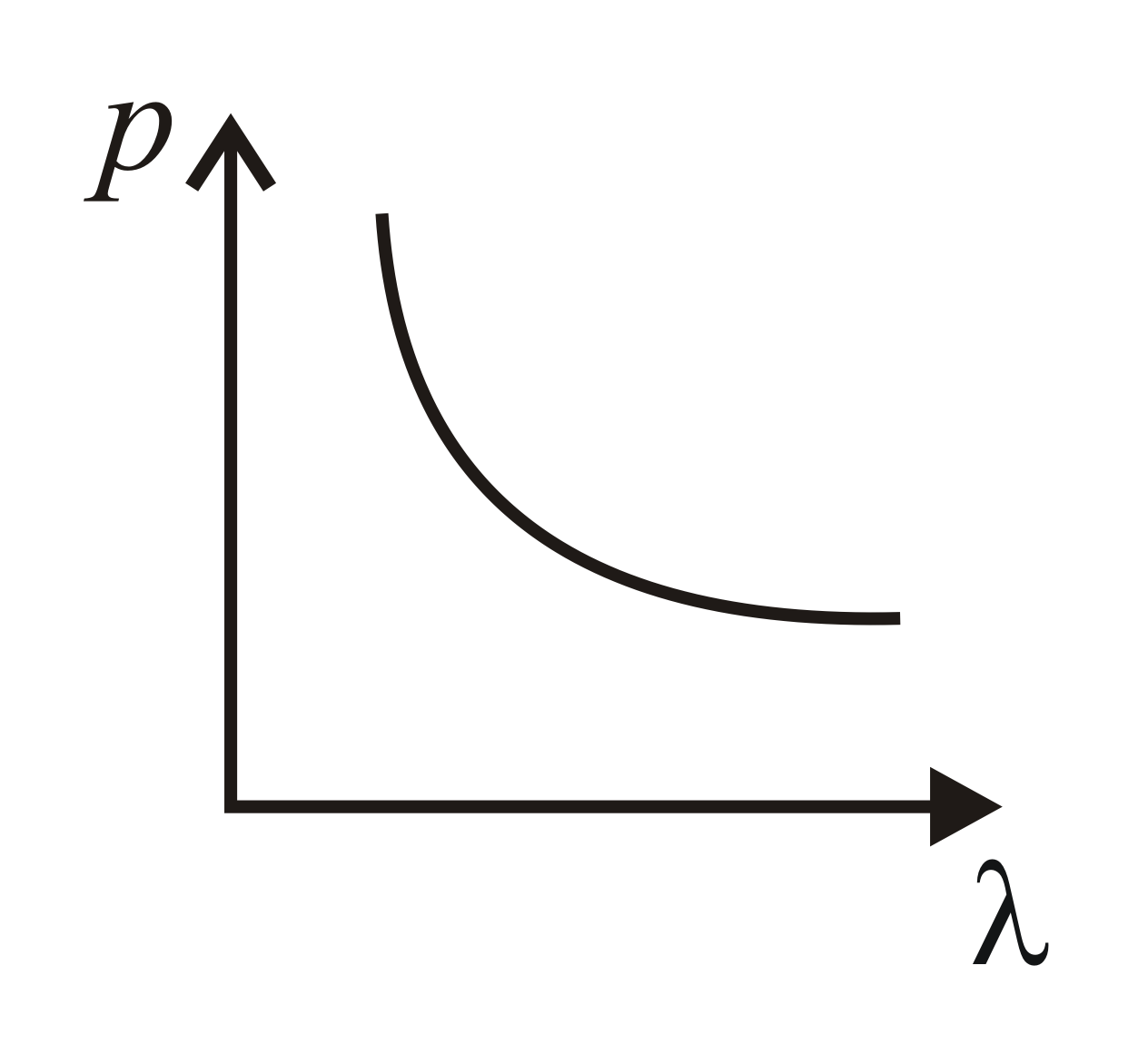
40% studentsanswered this correctly

Important Questions on Electrons, Photons, Photoelectric Effect and X-Rays
EASY
JEE Main
IMPORTANT
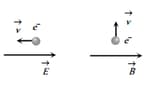
An electron is moving through a field. It is moving opposite to an electric field, perpendicular to a magnetic field as shown. For each situation, the de Broglie wavelength of the electron is:
EASY
JEE Main
IMPORTANT
The energy that should be added to an electron to reduce its de Broglie wavelength from to is:
HARD
JEE Main
IMPORTANT
A particle of mass at rest decays into two particles of masses and , having non-zero velocities. The ratio of the de Broglie wavelengths of the particles, is
MEDIUM
JEE Main
IMPORTANT
The ratio of de Broglie wavelengths of molecules of hydrogen and helium which are at temperatures of and respectively is:
EASY
JEE Main
IMPORTANT
The potential energy of a particle of mass, is given by:
and are the de Broglie wavelengths of the particle, when and respectively. If the total energy of particle is , the ratio will be:
HARD
JEE Main
IMPORTANT
A particle moves in a closed orbit around the origin, due to a force which is directed towards the origin. The de Broglie wavelength of the particle varies cyclically between two values and with . Which of the following statements are true?
MEDIUM
JEE Main
IMPORTANT
A particle moves in a closed orbit around the origin, due to a force which is directed towards the origin. The de Broglie wavelength of the particle varies cyclically between two values and with . Which of the following statements are true?
MEDIUM
JEE Main
IMPORTANT
Two particles and of masses and have the same de Broglie wavelength. Then,
