EASY
Earn 100
Which of the following gas turns lime water milky?
(a)Carbon dioxide
(b)Nitrogen dioxide
(c)Sulphur dioxide
(d)Water vapour
50% studentsanswered this correctly
Important Questions on Nature and Behaviour of Matter
HARD
MEDIUM
MEDIUM
MEDIUM
EASY
What happens when: (write chemical equation only).
(ii) Sulphuric acid reacts with caustic soda
MEDIUM
MEDIUM
EASY
EASY
What happens when: (write chemical equation only).
(i) Zinc reacts with dilute sulphuric acid
EASY
MEDIUM
EASY
Give reasons for the following statements: (i) Milk of magnesia is used for the treatment of acidity in the stomach.
MEDIUM
HARD
MEDIUM
Which gas is produced during the reaction in the test tube? How does this gas react with calcium hydroxide/lime water?
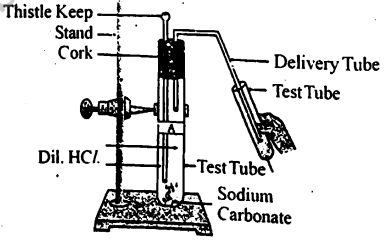
EASY
EASY
Give balanced chemical equation for following reaction-
MEDIUM
EASY
MEDIUM

