MEDIUM
JEE Main
IMPORTANT
Earn 100
Which of the following graphs correctly represents the variations?
(a)
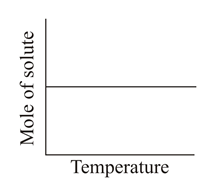
(b)
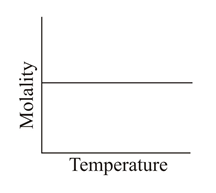
(c)
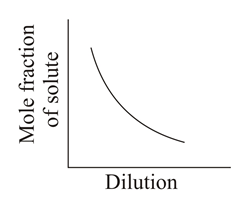
(d)
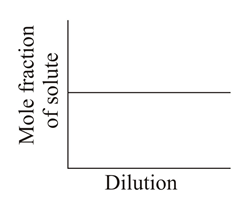
50% studentsanswered this correctly

Important Questions on Some Basic Concepts, Mole Concept and Stoichiometry
MEDIUM
JEE Main
IMPORTANT
Which of the following concentration is correct for mass percent of sulphuric acid (density: )?
MEDIUM
JEE Main
IMPORTANT
will be neutralized by:
HARD
JEE Main
IMPORTANT
In water, of an impure sample of oxalate was dissolved, making the solution to . of this required of solution on titration. Calculate the percentage of pure oxalate in the sample.
HARD
JEE Main
IMPORTANT
Both and can be used to titrate . If in a given titration, of were used, then what volume of solution will be consumed for the same titration in acidic medium?
HARD
JEE Main
IMPORTANT
An impure sample of , that has moist clay as impurity, on heating, loses of its weight of water. of the sample shows a loss in weight by . Calculate the percentage of in the sample.
HARD
JEE Main
IMPORTANT
Calculate the weight of quick lime and soda ash required to soften one million litres of hard water containing dissolved salts as shown below:
.
HARD
JEE Main
IMPORTANT
A mixture contains and some impurities. of the mixture was heated and the gases released were passed in pyrogallol. The weight of pyrogallol is increased by . The residue left needs—for complete reaction— of . How much and is present in the mixture?
HARD
JEE Main
IMPORTANT
A mixture contains of , of and of in a litre volume vessel. of the mixture is titrated with . What is the titer value, if phenolphthalein is added as the first indicator?
