MEDIUM
JEE Main
IMPORTANT
Earn 100
Which of the following graphs incorrectly represents the variation of molar conductance with dilution for a strong electrolyte?
(a)
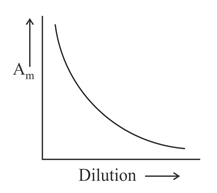
(b)
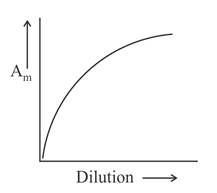
(c)
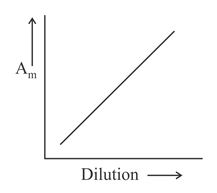
(d)
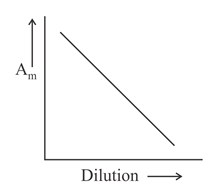
100% studentsanswered this correctly

Important Questions on Electrochemistry
MEDIUM
JEE Main
IMPORTANT
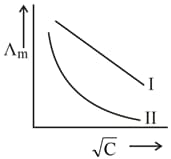
Above plot represents the variation of molar conductivity against (where, molar concentration of the electrolyte). Select the incorrect options among the following.
MEDIUM
JEE Main
IMPORTANT
An aqueous solution of copper sulphate is electrolysed using platinum electrodes in one case and copper electrodes in another case. Will the products of electrolysis be the same or different. Give reason?
EASY
JEE Main
IMPORTANT
State the products of electrolysis obtained on the cathode and the anode in the following case:
A dilute solution of with platinum electrodes.
EASY
JEE Main
IMPORTANT
State the products of electrolysis obtained on the cathode and the anode in the following case:
An aqueous solution of with silver electrodes.
MEDIUM
JEE Main
IMPORTANT
A current of Ampere is passed for one hour between nickel electrodes in of solution. What will be the molarity of the solution at the end of the electrolysis?
EASY
JEE Main
IMPORTANT
On electrolysis of an aqueous solution of , why is and not liberated at the cathode?
HARD
JEE Main
IMPORTANT
One faraday of electricity deposits one mole of from the molten salt but mole of from an aluminium salt. Why?
MEDIUM
JEE Main
IMPORTANT
In the electrolysis of an aqueous solution of sodium sulphate, of oxygen at STP was liberated at anode. The volume of hydrogen at STP liberated at cathode would be:
