EASY
AP EAPCET
IMPORTANT
Earn 100
Which of the following graphs represents Charle's law?
(a)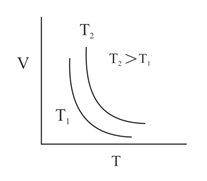

(b)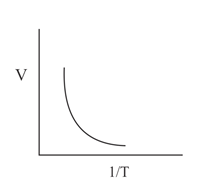

(c)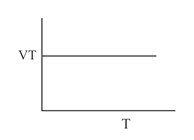

(d)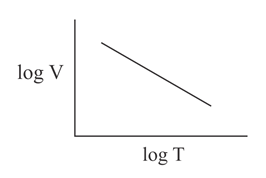

50% studentsanswered this correctly

Important Questions on States of Matter : Gases and Liquids
MEDIUM
AP EAPCET
IMPORTANT
The temperature to which the open vessel at is to be heated to expel of the air in it is (Assume (i) volume of the vessel remains constant and (ii) ideal behaviour for all gases present in air)
MEDIUM
AP EAPCET
IMPORTANT
At constant temperature, when a bulb of volume containing an ideal gas was connected to another evacuated bulb the pressure fell down by of bulb A's initial pressure. Then, find the volume of bulb
MEDIUM
AP EAPCET
IMPORTANT
Calculate the volume occupied by of water vapour at and bar pressure.
MEDIUM
AP EAPCET
IMPORTANT
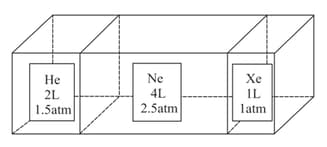
Consider the composite system, which is held at shown in the figure. Assuming ideal gas behavior, calculate the total pressure if the barriers separating the compartments are removed. Assume that the volume of the barriers is negligible.
MEDIUM
AP EAPCET
IMPORTANT
Calculate the density of gas at and pressure.
MEDIUM
AP EAPCET
IMPORTANT
An evacuated glass vessel weighs when empty, when filled with a liquid of density and when filled with an ideal gas at at Then find the molar mass of the ideal gas.
EASY
AP EAPCET
IMPORTANT
The internal energy of one mole of an ideal gas is given by the expression:
MEDIUM
AP EAPCET
IMPORTANT
At what temperature in, the most probable velocity of ozone gas is equal to RMS velocity of oxygen gas at
