Which of the following has the highest molar conductivity?
Important Questions on Redox Reactions and Electrochemistry
(where the constant B is positive)
Given below are two statements:
Statement I : The limiting molar conductivity of (strong electrolyte) is higher compared to that of $\mathrm{CH}_{3} \mathrm{COOH}$ (weak electrolyte).
Statement II : Molar conductivity decreases with decrease in concentration of electrolyte.
In the light of the above statements, choose the most appropriate answer from the options given below:
The variation of molar conductively with concentration of an electrolyte (X) in aqueous solution is shown in the given figure.
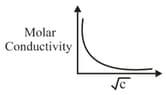
The electrolyte X is :
Given below are two statements:
Statement I: For , molar conductivity increases steeply with dilution.
Statement II: For carbonic acid, molar conductivity increases slowly with dilution. In the light of the above statements, choose the correct answer from the options given below
Match List - I with List - II :
| List - I (Parameter) |
List - II (Unit) | ||
| (a) | Cell constant | (i) | |
| (b) | Molar conductivity | (ii) | Dimensionless |
| (c) | Conductivity | (iii) | |
| (d) | Degree of dissociation of electrolyte | (iv) |
: Conductivity always increases with decreases in the concentration of electrolyte.
: Molar conductivity always increases with decreases in the concentration of electrolyte.
The correct option among the following

