HARD
Earn 100
Which of the following is/are correct for the first order reaction? (a is the initial concentration of reactant, is concentration of the reactant reacted and is time)
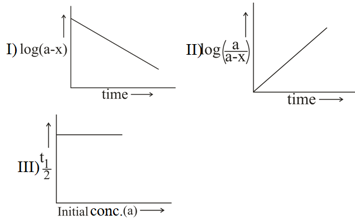

(a) and only
(b) and only
(c) and only
(d) and only
100% studentsanswered this correctly
Important Questions on Chemical Kinetics
MEDIUM
MEDIUM
HARD
MEDIUM
EASY
MEDIUM
The given plots represent the variation of the concentration of a reactant with time for two different reactions . The respective orders of the reaction are
(i) 
(ii) 
EASY
MEDIUM
EASY
EASY
MEDIUM
HARD
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
is zero
EASY
EASY

