MEDIUM
Earn 100
Which of the following is a strong electrolyte?
Important Questions on Ionic Equilibria
EASY
Write short note on following-
Strong Electrolyte
EASY
Write short note on following-
Weak Electrolyte
EASY
HARD
Differentiate between the following pair based on the information given in the brackets.
- Conductor and electrolyte (conducting particles)
EASY
HARD
EASY
MEDIUM
EASY
EASY
An electrolyte which completely dissociates into ions is:
MEDIUM
MEDIUM
What are electrolytes?
EASY
MEDIUM
EASY
EASY
Label the conjugate acid-base pair in the following reactions:
a. .
b. .
HARD
For which of the following solute, plot is:
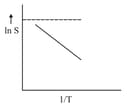
when represents the enthalpy of the solution?
MEDIUM
MEDIUM
| Species | Conjugate acid | Conjugate base |
|---|---|---|
MEDIUM

